Heparin
This is a mucopolysaccharide that inhibits blood clotting. Chemically, it is a linear polysaccharide glycosaminoglycan (see Glycosaminoglycans) with sulfogroups attached to it. Heparins are similar to heparan sulfates, but they contain fewer N-acetyl groups, more sulfogroups, and a larger molecular weight. The most commonly recurring disaccharide units are composed of 2-O-sulfoiduronic acid linked by an α-1-4 glycosidic bond to D-galactose or N-sulfo-glucosamine (IdoA(2S)-GlcNS(6S)). The natural molecular weight of heparin ranges from 3-30 kD. It is a molecule with the highest negative charge. Under physiological conditions, sulfogroup esters and amides are deprotonated, interact with positively charged ions, and form salts. Heparin is stored and synthesized in the secretory granules of liver, lung, and other tissue mast cells, released into the bloodstream when tissues are damaged. It is believed that heparin at the site of injury prevents bacteria and foreign substances from entering. It is a potent anticoagulant. Heparin binds to the inhibitor enzyme antithrombin III (AT), activating it. Active AT inactivates thrombin and other proteases involved in blood clotting. Heparin and its low molecular weight derivatives are used for the treatment and prevention of thrombosis and thrombotic embolisms. The activity of heparin is expressed in heparin units. One heparin unit is the amount of heparin equivalent to 0.002 mg of pure heparin, which protects 1 ml of cat blood from clotting for 24 hours at 0 °C. Heparin does not cross the placenta, so it can be used during pregnancy.
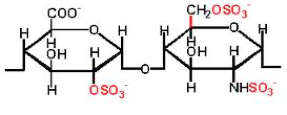
Heparin
Source | Glossary of Most Commonly Used Biomedical Terms and Concepts | Lithuanian University of Health Sciences | Academician Professor Antanas Praškevičius, Professor Laima Ivanovienė
