Prostaglandins
There are biologically very active substances synthesized in tissues from polyunsaturated fatty acids. Prostaglandins were first isolated from extracts of the prostate gland. Later it was noticed that they are widely spread in organisms and plants. Prostaglandins and enzymes catalyzing their biosynthesis are not found only in human erythrocytes. It should be noted that they are more present in reproductive system organs and tissues. Arachidonic acid (C-20:4, w-6) is used for prostaglandin biosynthesis, obtained from food or synthesized de novo in the body from essential fatty acids by cleaving membrane phospholipids. Arachidonic acid is released from phospholipids by the phospholipase A2 enzyme present in the membrane. Indirectly, this acid is also released by phospholipase C. Phospholipase A is a specific enzyme that hydrolyzes the ester bond at the 2nd carbon atom of glycerophosphate, having a special center for attaching arachidonic acid.
Arachidonic acid metabolism occurs through three pathways: cyclooxygenase pathway – during which prostaglandins and thromboxanes are formed; lipoxygenase pathway – leukotrienes are formed; and cytochrome P450 pathway – eicosanoids are synthesized, without a specific established function. The half-life of many eicosanoids is short (from a few seconds to a few minutes), they are quickly inactivated and excreted from the body in urine. The cyclooxygenase of fatty acids is part of the prostaglandin synthase enzyme complex. The enzyme can act as cyclooxygenase and peroxidase. It has two catalytic centers. There are two types of cyclooxygenase in the body: PGH2 synthase – cyclooxygenase 1, synthesized at a constant rate; and cyclooxygenase 2, whose synthesis increases during inflammation and is induced by corresponding mediators (cytokines). Both types of cyclooxygenase catalyze the insertion of 4 carbon atoms into arachidonic acid and the formation of a five-membered ring. Initially, an unstable hydroperoxide compound called PGG2 is formed. The hydroperoxide is quickly reduced to a hydroxyl group at the 15th carbon atom, forming PGH2. Until the formation of PGH2, the synthesis of all prostaglandins is the same. The final prostaglandin synthesis products in different organs and tissues are different.
Considering the different oxygen substitutes and their numbers, as well as the distribution of double bonds in the cyclopentane ring, prostaglandins are divided into several types, denoted by Latin letters A, B, C, D, E, F, G, H:
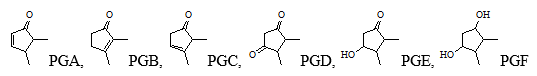
Each type includes several series, which differ in the number of double bonds in the lateral molecule part and are denoted by numerical indices (e.g., PGE1, PGE2, PGE3). The double bond between C-13 and C-14 is of trans configuration, while others are mostly of cis- configuration. The position of the -OH group in the cyclopentane ring can also vary. All natural prostaglandins have an a configuration.
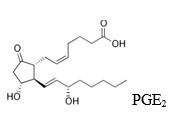
All prostaglandins have 20 carbon atoms.
In the vascular endothelium, a special prostaglandin I2 (PGI2) enzyme prostaglandin-1-synthase (EC 5.3.99.4) known as prostacyclin is formed from PGG2 endoperoxide. It is a strong inhibitor of platelet aggregation. Thromboxanes and prostacyclins are antagonists in terms of platelet aggregation. The ratio of thromboxane (TXA2) to prostacyclin (PGI2) mostly determines the possibility of thrombus formation on the vascular endothelium. Stable natural prostaglandins belong to the E and F types. Prostaglandins have various physiological effects. They manifest at very low prostaglandin concentrations of 10-9 mol/l. Prostaglandins act on smooth muscles, regulate blood flow to specific organs, blood pressure, ion transport across the membrane, modulate hormone activity. They mostly influence the functions of the cells in which they are formed. The nature of prostaglandin action depends on the cell type. They can be used as a therapeutic agent, for example, to prevent or terminate pregnancy, promote normal childbirth, treat stomach ulcers, inflammatory processes, relieve asthma attacks, and more. Prostaglandins increase cAMP levels in platelets, thyroid gland, corpus luteum, fetal bone tissue, anterior pituitary lobe, decrease cAMP levels in kidney tubules and adipose tissue. Prostaglandins PGI2, PGE2, and PGD2 dilate blood vessels and increase cAMP levels, reduce platelet and leukocyte aggregation, interleukin (IL-1a, IL-2) secretion, which, like cytokines, activate the immune system, T cell proliferation, and leukocyte migration. PGF2a constricts blood vessels, bronchi, smooth muscles. Prostaglandin analogs are used to treat certain diseases, for example, PGE1 and PGE2 structural analogs block histamine receptors (H2) on mucosal cells, inhibit gastric acid secretion, thus used to treat stomach and duodenal ulcers, PGE2 and PGF2a analogs contract uterine smooth muscles and are used to stop bleeding.
Source | Glossary of Most Commonly Used Biomedical Terms and Concepts | Lithuanian University of Health Sciences | Academician Professor Antanas Praškevičius, Professor Laima Ivanovienė
